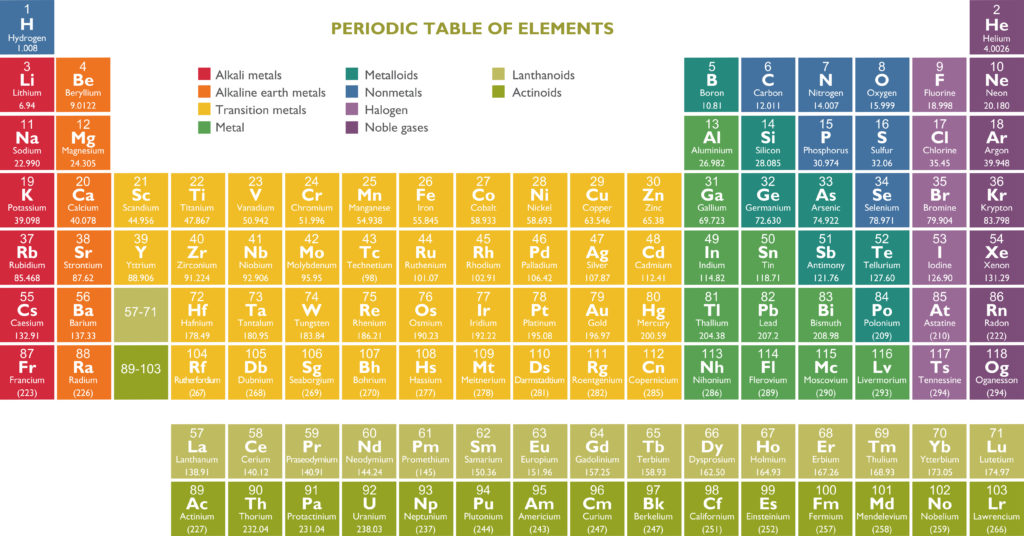
The Periodic Table is a table of all the elements we know about today. Elements are placed in order of atomic number ( number of protons in the nucleus ). The periodic table is a way of listing and organising elements.
The rows of the periodic table are called periods, and the columns are known as groups. Groups have similar physical and chemical properties.
The group an element belongs to corresponds to the number of electrons in its outer shell. Group 1 elements have 1 outer shell electron, group 2 has two outer shell electrons, and group 0 has full other shells with 8 electrons ( or 2 for Helium ).
How many elements are there in the Periodic Table?
The periodic table currently contains 118 different elements. Each square represents one element. Elements with similar properties are grouped together.
How are elements numbered in the Periodic Table?
Elements are numbered from left to right in order of how many protons their atoms contain. Hydrogen is the first element in the Periodic Table, as its atom contains one proton.
Metals are on the left of the table, metalloids are in the middle, and nonmetals are on the right.
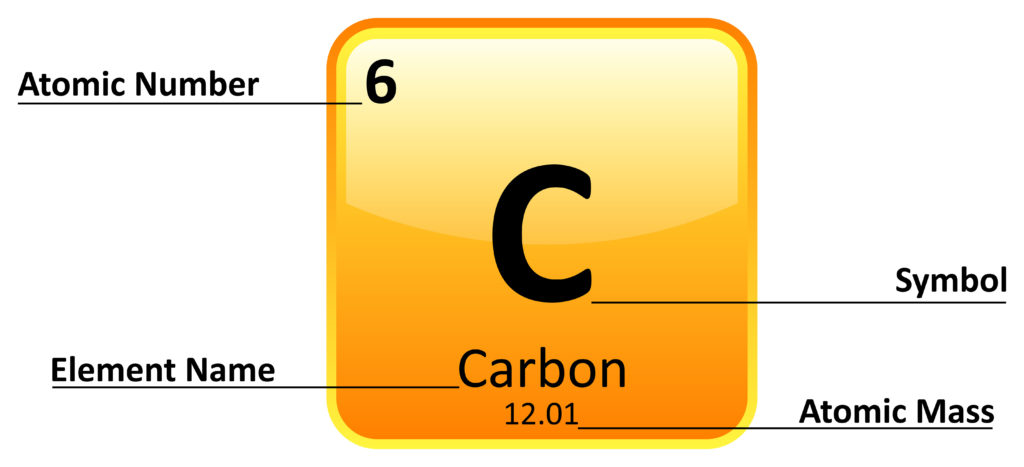
Each square of the periodic table contains information about the element.
Who invented the Periodic Table?
The Periodic Table was invented by Dmitri Mendeleev in 1869, who was the first scientist to put elements in order of atomic mass. Mendeleev was so clever at spotting patterns he left gaps for undiscovered elements.
The modern periodic table is slightly different to the one created by Mendeleev as it is ordered by atomic number, not atomic mass.
Chemical symbols that don’t look like their name
Each element has a chemical symbol that is the same in every language. Some are obvious, such as Li for Lithium, but some are harder to decipher. The first letter is always written as a capital, and the second is lowercase.
Tungsten has the symbol W – this is because tungsten is wolfram in German
Lead has the symbol Pb – this comes from the Latin word plumbum.
Mercury is Hg
Iron is Fe
Group 1 and 2 Elements
The red column is known as Group 1, and the orange column is Group 2. The elements in these groups are known as reactive metals. Pure sodium fizzes and shoots around when placed in water, potassium bursts into flame in water and caesium explodes when added to water! The reactivity of the elements increases as you go down the column.
Transition metals
The yellow block of elements in the centre of the table is known as transition metals. These elements are very useful as they conduct electricity and are mostly solid at room temperature.
Halogens – Group 7
Halogens are the elements in light purple. The halogen column is the only column to contain elements that are solids, liquids and gases. Fluorine is a yellow gas, bromine is a red liquid, and iodine is a purple solid.
Halogens react with group 1 metals to create compounds. One example of this is sodium reacting with chlorine to form sodium chloride.
Noble Gases – Group 0
These are the elements in dark purple. They are unreactive as they have a full outer shell of electrons and are very useful.
Noble gases are monatomic, they exist as single atoms.
Neon is used for neon signs, helium is used in balloons, argon is used in 3D printing, welding, lasers and has lots of other manufacturing uses.
Radioactive Elements
Many of the elements at the bottom of the table ( in green ) are radioactive.
Fun Facts about Elements
An element’s atomic number is the number of protons one atom of the element contains.
Radium was once used to paint glow-in-the-dark hands on clocks and watches.
Lithium is a metal but is so soft it can be cut with a knife.
Helium is lighter than air, which is why it is used to fill helium balloons.

Argon is used inside double-glazed windows as it doesn’t conduct heat.
Rocks that contain barium glow at night.
Mercury is the only transition metal that is a liquid at room temperature.
Sapphires are blue because of iron.
Bismuth is diamagnetic. Magnets repel it.
Neon is the least reactive element in the Periodic Table and is used in neon signs.
Titanium is very strong and very, very light!
Ideas for learning about the Periodic Table
Teach Beside Me has an amazing Periodic Table Battleships game.
Make paper plate atom models, we used these to learn about isotopes.

Sing the Periodic Table song, this one gets stuck in my head for weeks!
Last Updated on May 10, 2023 by Emma Vanstone
Leave a Reply