Isotopes are different forms of the same element. Isotopes of an element have the same number of protons and electrons but a different number of neutrons.
This means they have the same atomic number ( number of protons ) but a different mass number ( number of protons and neutrons ).
Paper Plate Isotope Models
These very simple paper plate models show the difference between Carbon-13 and Carbon -12. There is also a Carbon-14 which is radioactive as the extra 2 neutrons make it unstable.

Remember – elements always have the same atomic number ( number of protons ) but the mass number ( number of protons and neutrons ) can be different.

Examples of Isotopes
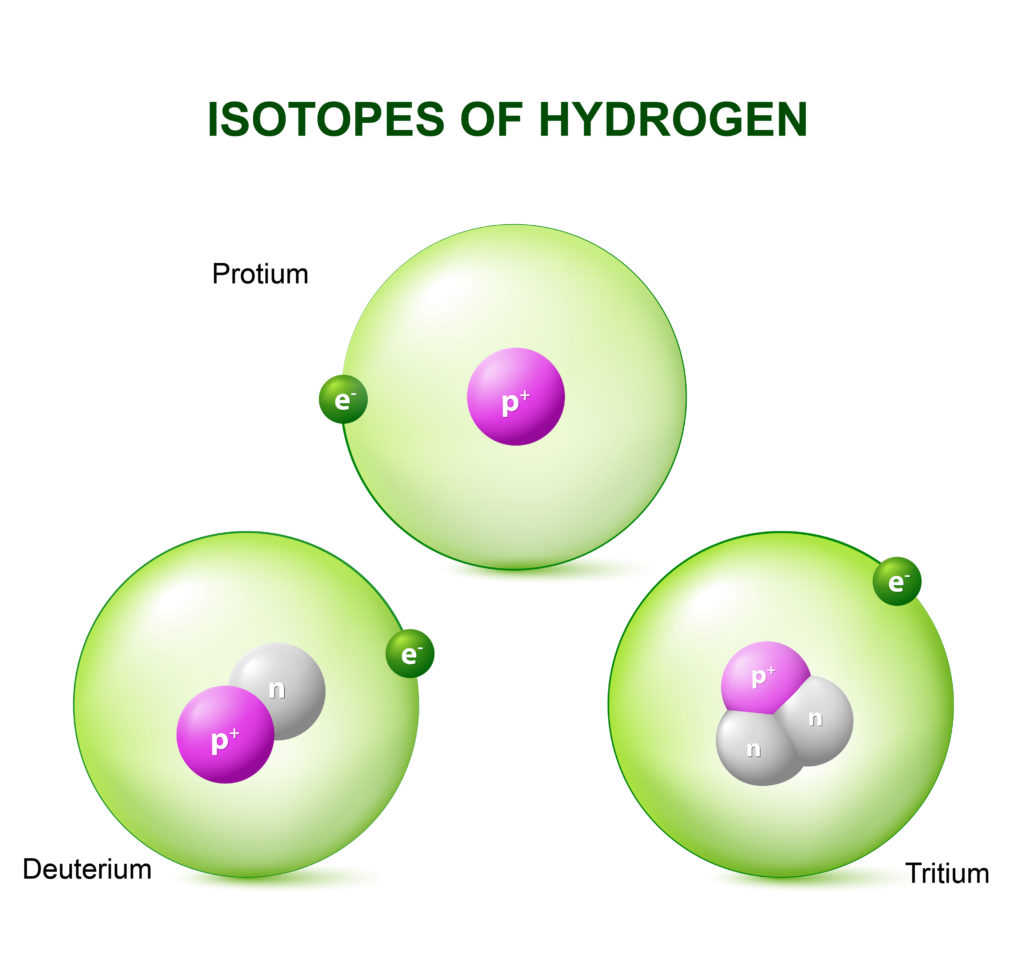
Isotopes of Hydrogen
Hydrogen -1
Hydrogen-2
Hydrogen-3
The most common form of hydrogen is Protium. Protium has no neutrons.
Deuterium has 1 neutron and Tritium has 2 neutrons.
Tritium is radioactive.
Facts about isotopes
Tin has the most stable isotopes of any element.
The mass of an isotope is the number of protons plus the number of neutrons.
Hydrogen is the only element whose isotopes have their own names!
Cesium and Xenon have the most naturally occurring isotopes with 36 each!
Unstable isotopes are radioactive.

Last Updated on August 6, 2022 by Emma Vanstone

Leave a Reply