Everything is made of atoms. Atoms are really, really small and are actually made up of even smaller particles called electrons, protons and neutrons.
Atoms are not all the same. They have different numbers of electrons, protons and neutrons.
Each different kind of atom makes up an element.
An atom consists of a positively charged nucleus surrounded by negatively charged electrons which move around the nucleus in orbits. Much of an atom is empty space!
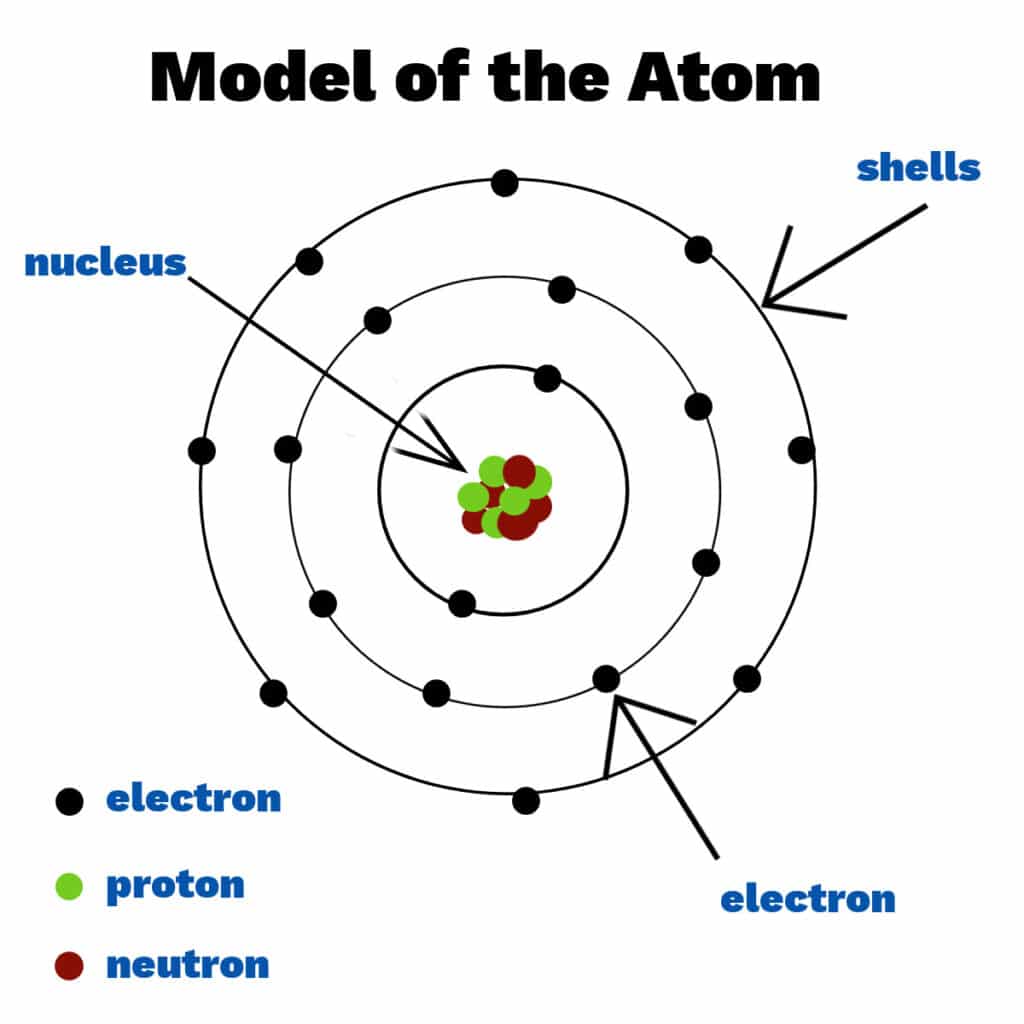
Electrons are negatively charged.
Protons are positively charged.
Neutrons have no charge.
Atoms have an overall neutral charge as the number of electrons is equal to the number of protons.
If an atom loses an electron, it becomes a positive ion. It has more protons than electrons.
If an atom gains an electron, it becomes a negative ion. It has more electrons than protons.
How big is an atom?
This video explains just how small atoms are brilliantly!
Atoms and Molecules
Atoms can join together to form a molecule.
For example, a molecule of oxygen gas consists of two oxygen atoms joined together. One fun way to learn about molecules and atoms is to make candy molecule models!

How many atoms make up a human?
An adult human is thought to contain around 7 Octillion atoms!!
What is atomic number?
Atoms of the same element have the same number of protons. The number of protons is the atomic number of the element.
Last Updated on June 18, 2023 by Emma Vanstone

Leave a Reply