Red cabbage indicator is a simple way to tell whether a substance is an acid or alkali.
Acids can be found in the food we eat, in our bodies and around the home. Some acids, especially those found in cleaning products, can be very harmful, so take care when using them and always read their hazard labels.
Alkalis are a group of chemicals that react with acids. Substances such as soap are alkalis and bicarbonate of soda, which we use in baking, and also harmful things like bleach are alkalis.
You can make a straightforward red cabbage pH indicator to determine whether a substance is an acid or alkali. This activity is a great way to introduce the pH scale to kids!
How to make a red cabbage pH indicator
What is a pH indicator?
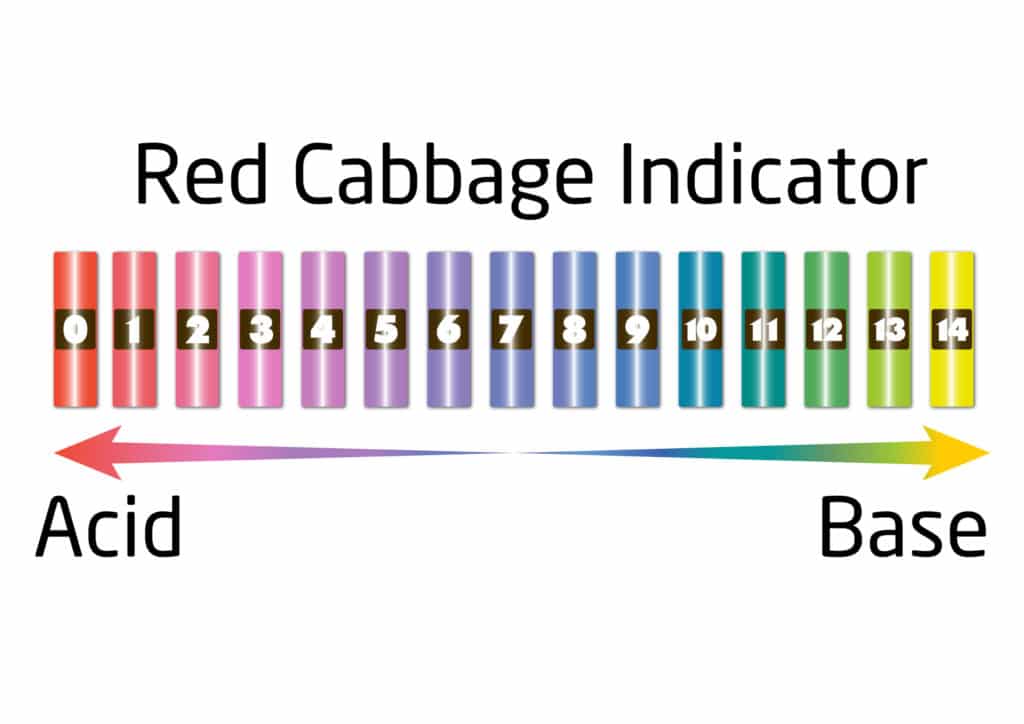
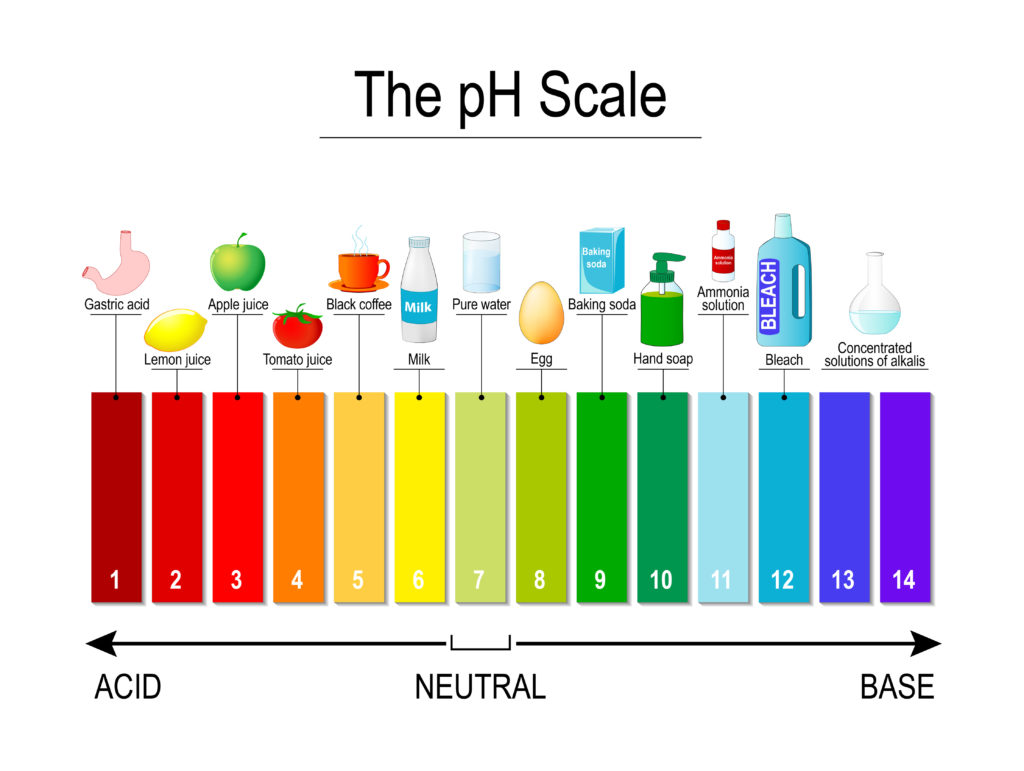
We use a substance called an indicator to test the pH of something. An indicator will change colour in the presence of an acid or alkali. The pH scale goes from 0 to 14. A substance with a pH of 0 is a strong acid, pH 14 is a strong alkali, and pH 7 is neutral.
You can make an indicator using red cabbage.
What you need to make a red cabbage indicator
- Red cabbage – chopped
- Water
- A saucepan
- A sieve
- Cups or small containers
- Different substances to test – baking soda, vinegar, lemon juice and lime juice all work well.
Method
Place the chopped cabbage into the pan and cover it with water.
Simmer for 10 minutes.
Sieve the water and cabbage into a jug – you will notice that the cabbage liquid is very purple.
Leave to cool for about 30 minutes.
Add a small amount of each test substance to a separate cup or container, and try to keep the amount of test substance the same.
Use a pipette to drop about 20ml of red cabbage indicator into each cup and record the colour the indicator changes to.
Safety note
Wear safety goggles if using strong acids/bases.
An adult should help with the chopping and heating of the cabbage.
Red Cabbage Indicator pH Colours
Universal Indicator pH colours
Notice that the colours for acids and alkalis are different when using a universal indicator.
Results
When using the red cabbage indicator, the colour of the liquid will change from purple to red if it is an acid and from purple to green if it is an alkali. The different shades of colour will depend on the strength of the acid or alkali. The substance is said to be neutral if there is no colour change.
How does the pH indicator work?
Acids and bases are opposites; acids have a low pH, and bases have a high pH.
Red cabbage contains a pigment called anthocyanin, which is what changes colour.
More Red Cabbage Indicator Experiments
Make colour changing fizzy potions!

Make your pH test strips by soaking filter paper in red cabbage indicator and leaving it to dry. Once dry, cut the filter paper into strips and dip it into test substances. Try testing milk, fizzy drinks or soap. Can you predict their pH before testing?
Try using beetroot juice instead of red cabbage; which works best?
You could also try blowing into the indicator. What happens?
A pH indicator can also be made from red poinsettia leaves!
What happens when you blow into the red cabbage indicator?
The indicator should turn red as the carbon dioxide you breathe out reacts with the water to form carbonic acid.

Last Updated on October 16, 2023 by Emma Vanstone




Leave a Reply