Freshwater freezes at 0°C. The presence of salt lowers the freezing point, and the higher the salt content in the water, the lower the freezing point gets.
This is why salt is often used to grit icy roads in winter. It slows down water freezing on roads, making driving safer.
Seawater contains salt. It will freeze, but it requires lower temperatures than freshwater. Usually, only very cold parts of the sea, such as the Arctic and Antarctic, are cold enough for sea water to freeze.

Seawater freezes at around -1.8°C.
Why is seawater salty?
Salt in seawater is mostly caused by mineral ions washing into the water. Carbon dioxide in the air dissolves into rain, making it acidic. The acidic rain lands on rocks and starts to erode them, releasing mineral salts ( including sodium and chloride ) into the sea. Sodium Chloride is the chemical name for the salt we eat!
The Dead Sea is an example of a body of water that has become very salty. It’s so salty that visitors can sit on the surface!

Did you know?
Blocks of frozen seawater are known as floes.
Humans cannot drink salt water.
When seawater freezes, the salt molecules are pushed below the surface of the ice. This means polar ice is actually freshwater!
Does salty water freeze science investigation
You can find out if salty water freezes in your kitchen! This simple hands-on science activity takes just a couple of hours and only requires a few basic supplies.
You’ll need
Two small, empty plastic bottles or small bowls
Water
Salt
Freezer
Instructions
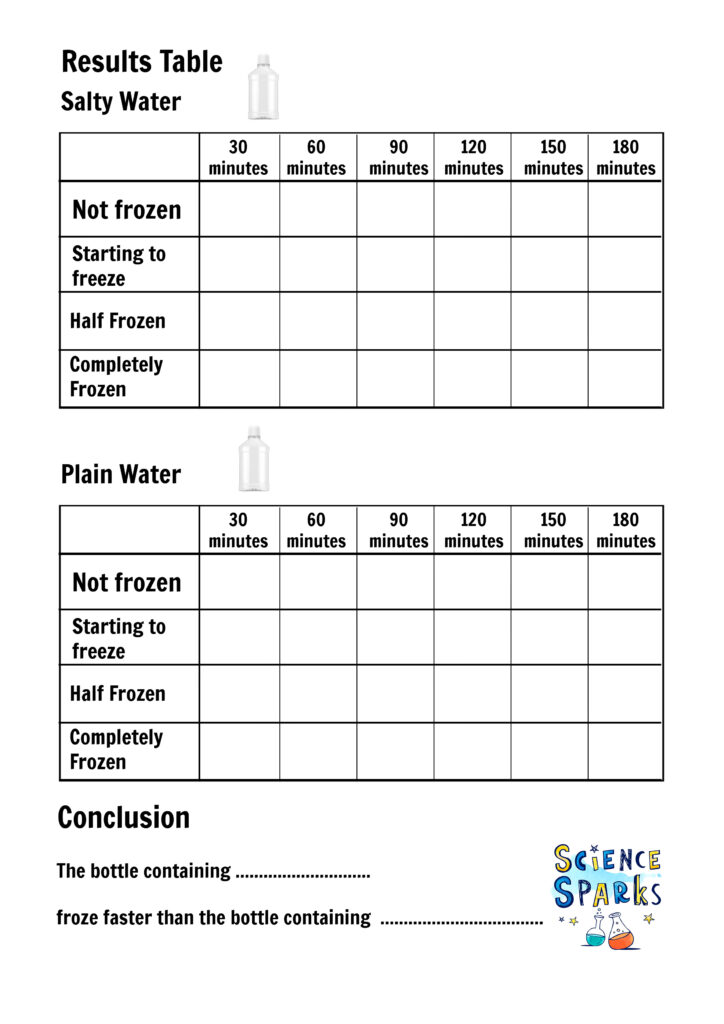
Place the same amount of water into each bottle.
Add two tablespoons of salt to one bottle and label this salt water.
Label the second bottle, plain water.
Place both bottles in a freezer and check them every 30 minutes.
You should find the salty water freezes more slowly than the plain water.
Does salty water freeze? Investigation Template
My handy experiment template guides you through the investigation with easy-to-follow instructions and space for results.


More science experiments using salt
Use salt to lift a piece of ice with string. In this clever trick, the salt melts the ice around the string. The ice then refreezes over the string, allowing the ice cube to be lifted.
Find out how to cool a drink with ice and salt.
Make a salt lava lamp. This is a fun twist on the more traditional lava lamp.

Last Updated on May 16, 2024 by Emma Vanstone

Leave a Reply